POLYSTYRENE PARTICLE ADSORPTION ON FLAT SURFACES
Introduction
The adsorption of colloidal particles or macromolecules in an adsorbent surface is a common phenomenon in many fields of chemistry, biology and physics. The adsorption has many applications. In industry is used for separating substances in the manufacture of paints, etc.. A model system to study the adsorption process in a suspension of polystyrene spheres that are adsorbed on a glass surface. In this work we go further and add another factor to our system which is the probability of irreversible stuck between them.
Beyond the simplicity of the problem, the adsorption process is determined by the interplay of several phenomena: the Brownian motion of free particles, the gravitational force, hydrodynamic interactions mediated by solvent, and direct interaction between free particles and adsorbed, as well as between particles and walls. The irreversible adsorption takes us out of equilibrium configurations, which we studied here.
We used an aqueous solution of polystyrene spheres of volume fraction of 4 x 10-3. The diameter of the particles is s = 1.9mm. To induce the adsorption on the glass wall of the bottom we added NaCl to the suspension at different concentrations ranging from 10mm to 90mm in increments of 20mm. We also created a cell, which consists of a slide and 3 coverslips prepared as shown in Fig.

The systems are observed using an inverted light microscope focused on the first layer of adsorbed particles to the bottom of the cell. The initial time evolution of the process is recorded continuously to monitor the kinetics of adsorption, the late time evolution is recorded at different times after and before the system reached a constant number of particles adsorbed.
 |
 |
 |
 |
 |
10mM |
30mM |
50mM |
70mM |
90mM |
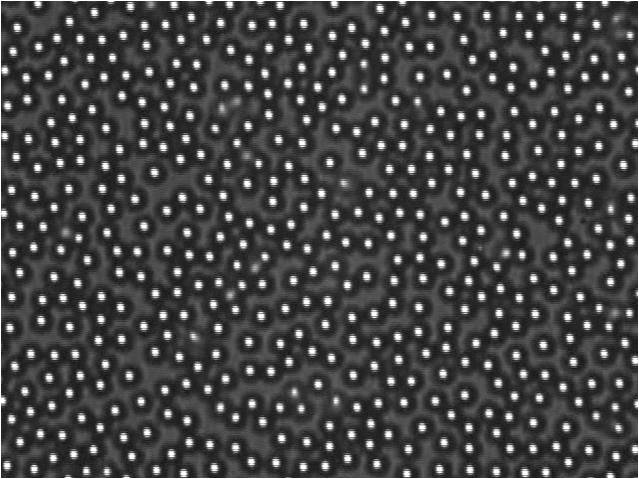
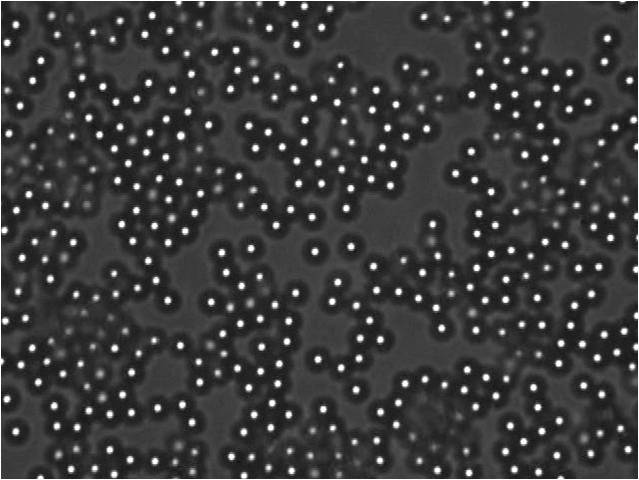
Fig1. Images of the distribution of particles in the glass wall of the fund, taken several days after the system completed. We note that there are more particles in the system 50mM. The 10mm and 30mm particles are not yet adsorbed to glass, and for 70mm and 90mm systems the particles are close together, but there are more empty spaces.
 |
Fig2. It shows the kinetics of adsorption, represented by the area fraction of particles versus time. |

The adsorption of particles depends strongly on the electrolyte amount in the system. The presence of electrolyte shielding the particle-wall electrostatic interaction, but also shielding the particle-particle interaction. Then the aggregation of particles is induced more strongly to higher concentrations of electrolyte. This explains the low shielding the area covered by adsorbed particles is lower, this increases as the electrolyte concentrations are increased but then the aggregation of particles reduces the area covered because the particles are attached to others before reaching the wall.